What's the difference between RO and DI water purification?
By Bob Applequist, Senior Application Specialist
On Tuesday, December 29, 2015
In Articles
People looking for the right Water Purification System often ask me, "What’s the difference between RO and DI water purification technologies?"
Here's the short answer: RO and DI filters use different physical reactions to clean water. Reverse Osmosis (RO), the reverse of osmosis, is often used to partially clean-up tap water to make it roughly 90% to 99% pure. Deionization (DI) filters exchange positive hydrogen and negative hydroxyl molecules for positive and negative contaminant molecules in water. DU filtering and other processes are sometimes referred to as "water polishing."
To answer the question more specifically, the individual technologies need to be understood.
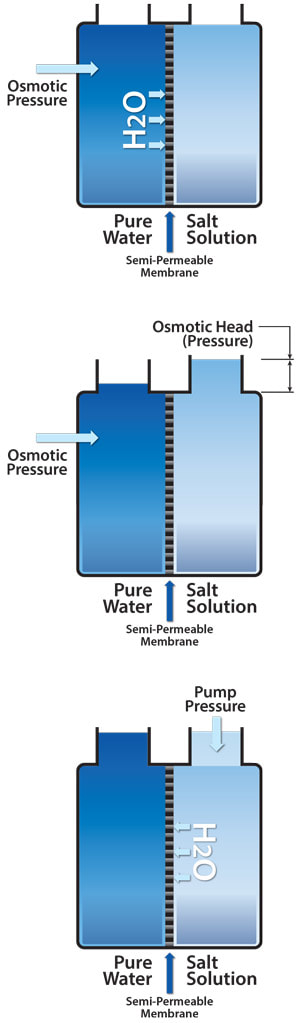
Reverse Osmosis (RO) is the opposite of a natural process simply called osmosis. Osmosis is the movement of water molecules across a semipermeable membrane. The process naturally moves water from a low ion concentration to a higher ion concentration across a semipermeable membrane. This natural process is used by our bodies to get water to our individual cells.
By applying pressure to the more concentrated (dirtier) side of a semipermeable membrane, water molecules are pushed back across the membrane to the less concentrated (cleaner) side, resulting in more purified water. This process is called Reverse Osmosis and can typically remove 90-99% of most contaminants. It is not perfect, but is a very cost effective technology; the RO Membrane can last for years if used properly. Reverse Osmosis is often used to partially clean-up tap water before any other purification technology is used to remove the remaining 1-10% of contaminants.
Deionization (DI) filters have many names: Ion Exchange, Strong Acid/Strong Base, Polishing, Nuclear Grade. Nuclear grade DI filters can remove Inorganic chemicals down to very low parts per billon (PPB) levels. This makes them excellent for producing Ultra-Purified (Type I, 18.2 Megohm) water. Water of this quality is used with the most sensitive laboratory analytical instruments, which are designed to detect chemicals at extremely low concentration levels.
Deionization filters function by exchanging positive hydrogen and negative hydroxyl molecules for the positive and negative contaminant molecules in the water. Positive chemicals, like calcium, exchange places with the hydrogen molecules and negative chemicals, like iodine, exchange places with the hydroxyl molecules. Over time, positive and negative contaminants in the water displace all the active hydrogen and hydroxyl molecules on the DI resin and the filter must be replaced. Regeneration of the deionization filter is possible, but only in an industrial environment setting.
Deionization is an on-demand process supplying purified water when needed. This is important because water at this extreme purity level degrades quickly. The nuclear grade deionization resin or polishing mixed bed resin removes almost all the inorganic contaminants in the water increasing the resistivity of the water to a maximum of 18.2 megohm-cm. However, deionization alone does not remove all types of contaminants like dissolved organic chemicals. Deionization filters are not physical filters with a pore size and cannot remove bacteria or particulates.
Quickly using up the purification capacity of deionization filters can be an expensive option for labs that choose to supply tap water to UltraPure (Type I) Polishing Systems.
There are several tests for identifying the purity level of water. The simplest test is a direct measurement of electrical conductivity or resistivity. Most inorganic chemicals are either negatively charged (anions) or positively charged (cations), and therefore transmit an electrical current when electrodes are inserted in the water. The more ions present, the greater the conductivity, or the lower the resistivity of the sample water.
Conductivity is expressed in microsiemens/cm and is used to measure water with a large number of ions present. Resistivity is expressed in megohms-cm and is used in the measurement of water with few ions. Conductivity and resistivity are mathematical reciprocals of each other. Therefore, at 25° C, 18.2 megohm water, which is the highest purity water obtainable also, has a conductivity of 0.055 microsiemen/cm.
By Bob Applequist, Senior Application Specialist
On Tuesday, December 29, 2015
In Articles
People looking for the right Water Purification System often ask me, "What’s the difference between RO and DI water purification technologies?"
Here's the short answer: RO and DI filters use different physical reactions to clean water. Reverse Osmosis (RO), the reverse of osmosis, is often used to partially clean-up tap water to make it roughly 90% to 99% pure. Deionization (DI) filters exchange positive hydrogen and negative hydroxyl molecules for positive and negative contaminant molecules in water. DU filtering and other processes are sometimes referred to as "water polishing."
To answer the question more specifically, the individual technologies need to be understood.
Reverse Osmosis (RO) is the opposite of a natural process simply called osmosis. Osmosis is the movement of water molecules across a semipermeable membrane. The process naturally moves water from a low ion concentration to a higher ion concentration across a semipermeable membrane. This natural process is used by our bodies to get water to our individual cells.
By applying pressure to the more concentrated (dirtier) side of a semipermeable membrane, water molecules are pushed back across the membrane to the less concentrated (cleaner) side, resulting in more purified water. This process is called Reverse Osmosis and can typically remove 90-99% of most contaminants. It is not perfect, but is a very cost effective technology; the RO Membrane can last for years if used properly. Reverse Osmosis is often used to partially clean-up tap water before any other purification technology is used to remove the remaining 1-10% of contaminants.
Deionization (DI) filters have many names: Ion Exchange, Strong Acid/Strong Base, Polishing, Nuclear Grade. Nuclear grade DI filters can remove Inorganic chemicals down to very low parts per billon (PPB) levels. This makes them excellent for producing Ultra-Purified (Type I, 18.2 Megohm) water. Water of this quality is used with the most sensitive laboratory analytical instruments, which are designed to detect chemicals at extremely low concentration levels.
Deionization filters function by exchanging positive hydrogen and negative hydroxyl molecules for the positive and negative contaminant molecules in the water. Positive chemicals, like calcium, exchange places with the hydrogen molecules and negative chemicals, like iodine, exchange places with the hydroxyl molecules. Over time, positive and negative contaminants in the water displace all the active hydrogen and hydroxyl molecules on the DI resin and the filter must be replaced. Regeneration of the deionization filter is possible, but only in an industrial environment setting.
Deionization is an on-demand process supplying purified water when needed. This is important because water at this extreme purity level degrades quickly. The nuclear grade deionization resin or polishing mixed bed resin removes almost all the inorganic contaminants in the water increasing the resistivity of the water to a maximum of 18.2 megohm-cm. However, deionization alone does not remove all types of contaminants like dissolved organic chemicals. Deionization filters are not physical filters with a pore size and cannot remove bacteria or particulates.
Quickly using up the purification capacity of deionization filters can be an expensive option for labs that choose to supply tap water to UltraPure (Type I) Polishing Systems.
There are several tests for identifying the purity level of water. The simplest test is a direct measurement of electrical conductivity or resistivity. Most inorganic chemicals are either negatively charged (anions) or positively charged (cations), and therefore transmit an electrical current when electrodes are inserted in the water. The more ions present, the greater the conductivity, or the lower the resistivity of the sample water.
Conductivity is expressed in microsiemens/cm and is used to measure water with a large number of ions present. Resistivity is expressed in megohms-cm and is used in the measurement of water with few ions. Conductivity and resistivity are mathematical reciprocals of each other. Therefore, at 25° C, 18.2 megohm water, which is the highest purity water obtainable also, has a conductivity of 0.055 microsiemen/cm.
